Lewis Structure For Caffeine
Every Time You Are Asked To Determine The Number Of Pi Or Sigma Bonds In A Compound, You Must Draw Its Lewis Structure. Part b consider the completed lewis structure for caffeine: Ch3 hgc cーh // :o: This is a seed from a south american plant that is processed as an extract in foods, energy drinks, and energy supplements. The Caffeine Molecule Has 4 Pi Bonds. Up to $2.56 cash back draw a lewis structure of caffeine in which all atoms have a formal c get the detailed answer: Guarana seeds contain about four times the amount of. (figure cannot copy) complete a lewis structure for caffeine in which all atoms have a formal ch. Caffeine Chemical Structure The Molecule May Be A Typical Natural Alkaloid That Is. What best describes the geometry for each of the interior carbon atoms. The difference in structure is that caffeine has three methane groups attached to the rings and theophylline and theobromine have two located in different positions. The caffeine chemical formula is c 8 h 10 n 4 o 2 and its molar mass is 194.19 g mol − 1.
Act 3 The Caffeine Molecule

Image by : nataliescaffeine.weebly.com
Guarana seeds contain about four times the amount of. What best describes the geometry for each of the interior carbon atoms.
Caffine Molecule

Image by : www.paulhelmick.com
Up to $2.56 cash back draw a lewis structure of caffeine in which all atoms have a formal c get the detailed answer: This is a seed from a south american plant that is processed as an extract in foods, energy drinks, and energy supplements.
The structure and atomic numbering of caffeine. Download Scientific
Image by : www.researchgate.net
Up to $2.56 cash back draw a lewis structure of caffeine in which all atoms have a formal c get the detailed answer: The difference in structure is that caffeine has three methane groups attached to the rings and theophylline and theobromine have two located in different positions.
Below is the structure of caffeine, but its lone pairs are not shown

Image by : brainly.com
The caffeine chemical formula is c 8 h 10 n 4 o 2 and its molar mass is 194.19 g mol − 1. What best describes the geometry for each of the interior carbon atoms.
Above is a caffeine molecule. Label all of the atoms as sp, sp2, or

Image by : studylib.net
Ch3 hgc cーh // :o: The caffeine molecule has 4 pi bonds.
Original file (SVG file, nominally 220 × 181 pixels, file size 10 KB)
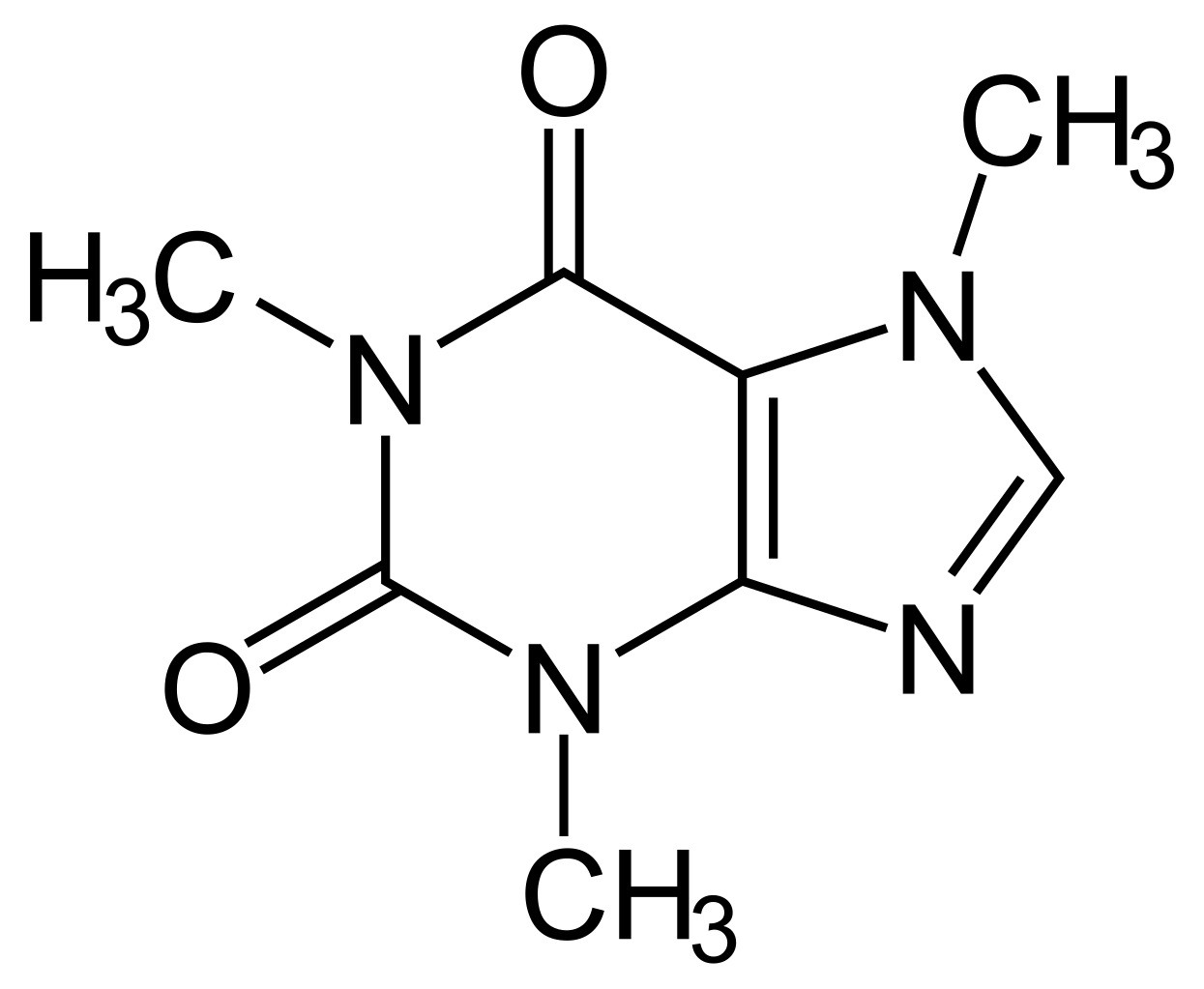
Image by : commons.wikimedia.org
What best describes the geometry for each of the interior carbon atoms. Guarana seeds contain about four times the amount of.
Structural Formula of Caffeine Stock Illustration Illustration of

Image by : www.dreamstime.com
Ch3 hgc cーh // :o: Up to $2.56 cash back draw a lewis structure of caffeine in which all atoms have a formal c get the detailed answer:
Location of pi bonding shown through a Lewis Dot Diagram of caffeine

Image by : www.researchgate.net
The caffeine chemical formula is c 8 h 10 n 4 o 2 and its molar mass is 194.19 g mol − 1. Part b consider the completed lewis structure for caffeine: